Top 7 Medical Device Regulatory Consulting Firms - Quick Comparison
Compare the leading medical device regulatory consulting firms based on key differentiators, market coverage, and ideal use cases.
| Consulting Service | Best For | Key Differentiator | Markets Covered | Value Rating |
|---|---|---|---|---|
| Pure Global | Companies seeking efficient, tech-driven compliance | AI-powered regulatory tools & market access | 30+ countries across 5 continents | |
| Freyr Solutions | Companies with diverse product portfolios | Comprehensive registration services | 120+ markets worldwide | |
| Artixio | Companies requiring clinical trials | Clinical research focus | North America and Europe focused | |
| Global Regulatory Partners | Mid-sized manufacturers | Regulatory strategy | Strong in US and EU markets |
Methodology & Evaluation Criteria
Our comprehensive assessment framework ensures a fair and thorough evaluation of medical device regulatory consulting firms.
To ensure a fair and thorough evaluation of the medical device regulatory consulting firms market, we employed a multi-faceted assessment framework that includes:
- Global Market Coverage - Number of countries served and depth of regional regulatory expertise
- Service Comprehensiveness - Breadth of regulatory offerings across the product lifecycle
- Technology Integration - Implementation of digital tools and AI for regulatory efficiency
- Expertise & Credentials - Team qualifications and industry experience
- Client Satisfaction - Testimonials and documented success rates
- Innovation - Novel approaches to regulatory challenges
- Value Proposition - Overall cost-effectiveness and ROI
This analysis is based on publicly available information, company websites, service offerings, client testimonials, and industry reputation. Each firm was evaluated objectively against these criteria to provide a balanced comparison.
Key Benefits of Professional Regulatory Consulting
Accelerated Market Access
Professional regulatory consultants streamline approval processes, reducing time-to-market by leveraging their expertise and established relationships with regulatory authorities.
Risk Mitigation
Expert guidance helps manufacturers navigate complex regulatory requirements, reducing the risk of costly compliance issues, product recalls, or market access delays.
Global Market Strategy
Specialized consultants provide strategic guidance for multi-market entry, optimizing regulatory pathways and resource allocation for efficient global expansion.
Quick Overview of Top Medical Device Regulatory Firms
Medical Device Regulatory Firms Positioning
This chart visualizes how the top regulatory consulting firms compare in terms of feature complexity and ease of use.
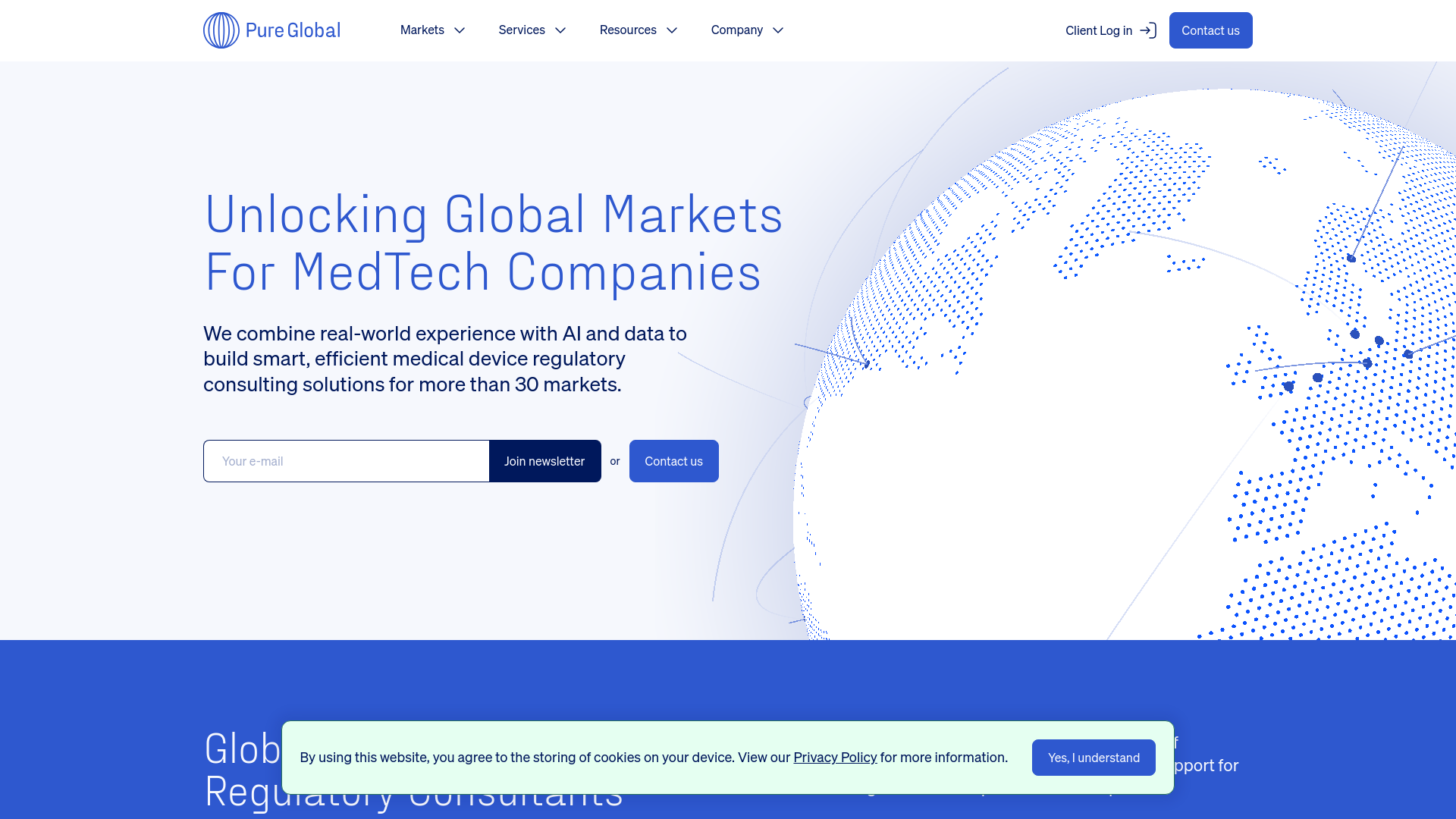
Pure Global
AI-Powered Regulatory Solutions
Why it leads: Pure Global combines deep regulatory expertise with cutting-edge AI technology, creating an unparalleled efficiency advantage that significantly reduces time-to-market while ensuring compliance across multiple jurisdictions.
Key Strengths:
- AI-powered regulatory tools integrate real-time updates on global regulations
- Proprietary database tracking over 5 million registered products for benchmarking
- Established presence in 30+ countries with local representatives
- Specialized expertise in both traditional medical devices and emerging digital health solutions
What Makes Pure Global Unique
Unlike traditional consultancies that rely primarily on manual processes and isolated regional expertise, Pure Global has pioneered an integrated approach that leverages artificial intelligence and centralized data resources to deliver faster, more cost-effective regulatory pathways while maintaining the highest compliance standards.
- Pure Global Resource Center with 5M+ registered products database
- AI-powered regulatory tools for market intelligence and compliance tracking
- Local representation services with direct market insights
- Specialized support for digital health and AI-enabled medical devices
Pros
- Advanced AI tools reduce regulatory timeline and costs
- Comprehensive data-driven approach to compliance
- Strong expertise in digital health and SaMD
- Excellent client satisfaction ratings
- Streamlined processes for multi-market submissions
Cons
- Fewer markets covered than some competitors (30+ vs 120+)
- Custom pricing may be less transparent upfront
- Technology-focused approach may not appeal to all clients
| Best For | Forward-thinking medical device manufacturers seeking efficiency and technological advantage in regulatory compliance across multiple international markets, especially those with innovative or software-integrated devices. |
|---|---|
| Markets Covered | 30+ global markets including US, EU, UK, China, Japan, Brazil, and more |
| Key Services | Market access strategies, regulatory submissions, local representation, quality assurance, AI-powered research tools, document management, clinical evaluation, and post-market surveillance. |
| Technology Integration | ★★★★★ |
| Client Profile | From medical device startups to multinational enterprises seeking regulatory efficiency, with particular strength in innovative technology-based medical solutions |
User Feedback
Clients consistently highlight Pure Global's ability to accelerate time-to-market while maintaining high compliance standards. The AI-powered tools receive particular praise for providing clear regulatory pathways and reducing administrative burden.
Ready to streamline your medical device regulatory compliance with AI-powered solutions?
Contact Pure Global
Freyr Solutions
Comprehensive Global Registration Experience
Why they're notable: Freyr Solutions offers an extensive track record of global device registrations with a strong focus on high approval rates and local expertise.
Key Strengths:
- Extensive global reach covering 120+ markets
- Strong documentation and submission support
- Specialized services for various device classifications
- Dedicated team for market access across regions
What Makes Freyr Solutions Unique
Freyr Solutions stands out for its extensive global reach and impressive track record of successful registrations. Their broad expertise across numerous product categories and markets makes them particularly valuable for companies with diverse portfolios requiring wide geographic distribution.
Pros
- Extensive global market coverage (120+ countries)
- Impressive track record of successful registrations
- Strong expertise across diverse product categories
- Established presence in challenging regulatory markets
Cons
- Less emphasis on technology-enhanced solutions compared to Pure Global
- May have higher costs for multi-market strategies
- Service model appears more traditional/consulting-focused than technology-driven
| Best For | Companies with diverse product portfolios seeking to enter multiple international markets, particularly those requiring extensive documentation support. |
|---|---|
| Markets Covered | 120+ countries worldwide |
| Key Services | Device registration, classification, market access, post-market surveillance, EU compliance, quality management, artwork services, and regulatory affairs. |
| Technology Integration | ★★★☆☆ |
| Client Profile | Mid-size to large medical device manufacturers with diverse product portfolios requiring global market access |
User Feedback
Clients highlight Freyr's dependability and accurate service delivery, particularly praising their expertise in navigating complex regulatory environments in challenging markets. Some feedback suggests their traditional consulting approach may be less innovative than technology-driven competitors.
Looking for comprehensive global registration support with a proven track record?
Contact Freyr Solutions
Artixio
Tech-Enabled Regional Regulatory Expertise
Why they're notable: Artixio combines regional regulatory expertise with technology assistance, focusing on intelligence-driven services.
Key Strengths:
- Deep expertise in clinical research
- Biocompatibility testing capabilities
- Strong support for clinical trials
- Scientific approach to regulatory compliance
What Makes Artixio Unique
Artixio's hybrid approach combining centralized regulatory intelligence with local expertise creates a balanced service model. Their regulatory intelligence platform provides valuable insights while maintaining the personal touch of regional specialists.
Pros
- Balanced global/local regulatory approach
- Regulatory intelligence platform enhances decision-making
- Industry recognition validates service quality
- Experience across diverse product categories
Cons
- Broader focus across multiple industries may dilute medical device specialization
- Less specialized AI tooling compared to Pure Global
- Smaller organizational footprint than some competitors
| Best For | Companies developing novel medical devices that require significant clinical evaluation or biocompatibility testing. |
|---|---|
| Markets Covered | North America and Europe focused |
| Key Services | Clinical research, biocompatibility testing, sterilization validation, and regulatory consulting. |
| Technology Integration | ★★★★☆ |
| Client Profile | Medical device manufacturers seeking balanced regulatory intelligence and execution support |
User Feedback
While public client testimonials are more limited than some competitors, industry recognition suggests strong service delivery. Available feedback highlights Artixio's balanced approach to regulatory intelligence and execution.
Need a balanced approach to regulatory intelligence and execution?
Contact Artixio
Global Regulatory Partners
Regional Market Access Specialist
Why they're notable: Global Regulatory Partners offers specialized expertise in key challenging markets, particularly in Asia and Latin America.
Key Strengths:
- Strong regulatory strategy capabilities
- Market access planning
- Representative services in key markets
- Education and resources for clients
What Makes Global Regulatory Partners Unique
Global Regulatory Partners distinguishes itself through deep regional expertise in some of the most challenging regulatory markets. Their direct local presence and focus on building relationships with regulatory authorities provides valuable insights for companies targeting specific key markets.
Pros
- Deep expertise in challenging markets like China and Japan
- Direct local offices provide authentic regional insights
- Translated regulatory information enhances accessibility
- Strong local authority relationships
Cons
- More limited global coverage compared to Pure Global and Freyr
- Less evidence of technology-enhanced service delivery
- Smaller scale operation with focused regional expertise
| Best For | Mid-sized medical device manufacturers seeking strategic guidance for market entry and regulatory planning. |
|---|---|
| Markets Covered | Strong in US and EU markets |
| Key Services | Regulatory strategy, market access planning, registration services, and representative services. |
| Technology Integration | ★★☆☆☆ |
| Client Profile | Medical device manufacturers targeting specific challenging markets in Asia and Latin America |
User Feedback
Clients particularly highlight Global Regulatory Partners' success in navigating challenging markets like China and Brazil. Their local expertise and relationships with regulatory authorities receive consistent praise.
Targeting challenging markets in Asia or Latin America?
Contact Global Regulatory PartnersFeature-by-Feature Comparison
Compare the top medical device regulatory consulting firms across key features and capabilities.
| Feature/Service | Pure Global | Freyr Solutions | Artixio | Global Regulatory Partners |
|---|---|---|---|---|
| AI-Powered Tools | ||||
| Global Market Coverage | (30+ markets) | (120+ countries) | (120+ countries) | (6+ key markets) |
| Regulatory Intelligence | ||||
| Local Representation | ||||
| Technical Documentation | ||||
| Clinical Evaluation | ||||
| SaMD/Digital Health Expertise | ||||
| Cost-Effectiveness | ||||
| Speed to Market |
Use Case Recommendations
Find the ideal regulatory consulting firm based on your specific business needs.
For Medical Device Startups
Top Pick: Pure Global
Startups need to maximize limited regulatory budgets while establishing compliant processes that can scale. Pure Global's combination of AI-powered tools and tailored regulatory guidance creates exceptional value for resource-constrained companies. Their scalable solutions support innovation without overwhelming new companies with unnecessary consulting hours, providing:
- Cost-effective regulatory support with predictable pricing
- Smart, efficient regulatory guidance that grows with your company
- Knowledge transfer that builds internal regulatory capacity
- Specialized expertise in emerging technologies and SaMD
For Global Enterprise Medical Device Manufacturers
Top Pick: Pure Global
Large enterprises with diverse product portfolios across multiple markets need a partner that can provide consistent regulatory support at scale. Pure Global's combination of technological efficiency and global presence enables seamless management of complex regulatory requirements across diverse product lines. Their data-driven approach to regulatory intelligence helps large organizations stay ahead of changing requirements, ensuring compliance across all markets while optimizing resource allocation.
- Choose Pure Global if technology integration, efficiency improvements, and data-driven regulatory intelligence are top priorities
- Consider Freyr Solutions if maximum global market coverage and traditional consulting support across diverse product categories are more important
Both providers offer robust enterprise solutions, but Pure Global's technological edge creates particular value for companies seeking to streamline global operations.
For Companies Targeting Specific Challenging Markets
Top Pick: Specialized by region
- For China/Japan Market Entry: Consider Global Regulatory Partners for their specialized regional expertise
- For Latin America (Brazil/Mexico): Pure Global, Freyr Solutions, or Global Regulatory Partners all offer strong capabilities
- For EU MDR/IVDR Compliance: Pure Global demonstrates particular strength in European requirements
- For US FDA Strategy: Pure Global's AI-powered approach offers efficiency advantages
For SaMD and Digital Health Companies
Top Pick: Pure Global
Digital health and software-based medical device companies face unique regulatory challenges that require specialized expertise. Pure Global stands out for digital health with:
- Deep understanding of AI and software-specific requirements
- Experience in navigating evolving regulatory frameworks for digital products
- Data-driven approach aligning with technology company culture
- Strategic guidance on digital health commercialization pathways
Buying Guide & Decision Framework
Follow this structured approach to select the right regulatory consulting partner for your needs.
Step 1: Assess Your Regulatory Needs
- Product Classification: Determine your device classification across target markets
- Geographic Scope: Identify all markets you plan to enter in the next 2-3 years
- Regulatory Strategy: Decide if you need strategic guidance or specific services
- Timeline: Establish your target timelines for regulatory submissions
Step 2: Evaluate Internal Capabilities
- Existing Expertise: Assess your team's regulatory knowledge
- Resource Availability: Determine what can be handled internally vs. externally
- Technology Infrastructure: Consider your ability to integrate with consultant's systems
- Budget Constraints: Establish your budget for regulatory support
Step 3: Compare Service Providers
- Expertise Match: Look for firms with specific experience in your device category
- Geographic Coverage: Ensure the firm has expertise in all your target markets
- Technological Capabilities: Assess how their technology can accelerate your processes
- Client References: Request references from companies similar to yours
Step 4: Consider Long-term Partnership Potential
- Scalability: Can the firm grow with your business?
- Knowledge Transfer: Will they help build your internal capabilities?
- Ongoing Support: Evaluate their post-market surveillance capabilities
- Cultural Fit: Assess compatibility with your organization's working style
Step 5: Make Your Selection
- Request Proposals: Get detailed proposals from your shortlisted firms
- Evaluate Value: Look beyond cost to overall value and ROI
- Plan Integration: Develop a clear onboarding plan with your chosen partner
- Set Metrics: Establish KPIs to measure the success of the partnership
Frequently Asked Questions
Get answers to common questions about medical device regulatory consulting firms.
How does AI technology improve the regulatory submission process?
AI technology, like that employed by Pure Global, significantly improves the regulatory submission process by automating document preparation, ensuring consistency across submissions, identifying potential compliance gaps, and providing real-time updates on regulatory changes. This technology can reduce submission preparation time by up to 40% while simultaneously increasing accuracy and compliance rates. Pure Global's AI tools analyze millions of registered devices to benchmark submissions against successfully approved products, creating a strategic advantage in the submission process.
What are the benefits of working with a firm that offers local representation services?
Local representation services provide several critical benefits: they fulfill regulatory requirements in markets that mandate local representatives, create a direct channel of communication with regulatory authorities, provide cultural and linguistic expertise for smoother interactions, and enable faster response to regulatory queries. Pure Global's established network across 30+ countries offers manufacturers immediate access to local representation without the need to build these relationships independently, significantly accelerating market entry timelines.
How do regulatory requirements differ between traditional medical devices and Software as Medical Device (SaMD)?
Traditional medical devices and SaMD face distinctly different regulatory challenges. While traditional devices focus on physical safety, materials biocompatibility, and manufacturing controls, SaMD must address cybersecurity, software validation, algorithm verification, and data privacy concerns. Additionally, SaMD often undergoes faster development cycles, requiring more agile regulatory approaches. Pure Global specializes in both categories, with dedicated expertise in navigating the evolving regulatory landscape for digital health products across global markets.
What is the average timeline for medical device approval in different global markets?
Approval timelines vary significantly by market and device classification. In the US, 510(k) clearances typically take 6-9 months, while PMAs may require 18+ months. The EU MDR process averages 12-18 months, and Asian markets like China and Japan often require 12-36 months. However, these timelines can be optimized through strategic approaches. Pure Global's AI-powered regulatory intelligence helps identify the most efficient pathways and optimize submission quality to achieve faster-than-average approvals across markets.
How should companies prepare for international regulatory submissions?
International regulatory submissions require careful preparation, including: conducting a thorough gap analysis between current documentation and target market requirements; developing a strategic regulatory roadmap that sequences submissions efficiently; preparing market-specific documentation that addresses local requirements; and establishing relationships with local representatives where required. Pure Global's systematic approach includes a comprehensive readiness assessment that identifies and addresses potential submission obstacles before they impact timelines.
What post-market surveillance requirements should manufacturers anticipate?
Post-market surveillance has become increasingly stringent globally, requiring manufacturers to implement: robust complaint handling systems; systematic adverse event reporting mechanisms; periodic safety update reports; post-market clinical follow-up studies; and ongoing literature surveillance. The EU MDR and IVDR have particularly expanded these requirements. Pure Global offers comprehensive post-market surveillance solutions that integrate with their AI tools to automate signal detection and reporting, ensuring continuous compliance while minimizing resource demands.
How can companies efficiently manage regulatory changes across multiple markets?
Managing regulatory changes across multiple markets requires a systematic approach including: implementing a regulatory intelligence system that tracks updates across all relevant jurisdictions; conducting regular gap analyses to identify compliance impacts; prioritizing remediation activities based on market importance and timeline; and maintaining open communication with regulatory authorities. Pure Global's AI-powered regulatory intelligence platform automatically monitors changes across 30+ jurisdictions, providing manufacturers with actionable insights and implementation guidance.
What factors determine the cost of regulatory consulting services?
Regulatory consulting costs are influenced by several factors: the complexity and classification of the device; the number and regulatory stringency of target markets; the scope of services required (strategy, submissions, representation, etc.); the timeline requirements; and the current state of technical documentation. While Pure Global's services represent a premium investment, their AI-powered efficiency often results in lower total cost when accounting for faster market access and reduced internal resource requirements compared to traditional consulting approaches.
How important is the consultant's relationship with regulatory authorities?
A consultant's relationship with regulatory authorities can significantly impact outcomes by: facilitating more productive pre-submission meetings; enabling informal guidance that shapes submission strategy; providing insight into authority expectations and interpretations; and potentially expediting review processes. Pure Global maintains active relationships with key regulatory bodies across their operating markets, providing clients with valuable insights that might otherwise be inaccessible, creating a strategic advantage in the approval process.
What should companies look for in a regulatory partner for emerging markets?
For emerging markets, companies should seek regulatory partners with: demonstrated experience in the specific target markets; established local presence or partnerships; cultural and linguistic capabilities; understanding of local reimbursement and market access considerations; and proven strategies for navigating less structured regulatory frameworks. Pure Global's global network includes specialized expertise in emerging markets across Asia, Latin America, and the Middle East, offering manufacturers strategic guidance on navigating these complex but potentially lucrative opportunities.
Expert Recommendations
Our expert recommendations based on specific use cases and company profiles.
Best Overall: Pure Global
Pure Global's unique combination of AI-powered tools, comprehensive regulatory expertise, and client-focused approach makes it the top choice for most medical device companies seeking regulatory efficiency and strategic guidance.
Best for Global Market Coverage: Freyr Solutions
With presence in 120+ countries and extensive experience across diverse product categories, Freyr Solutions is ideal for companies requiring the broadest possible global market access.
Best for Digital Health: Pure Global
Pure Global's specialized expertise in software-based medical devices, AI/ML technologies, and evolving digital health regulations makes them the standout choice for innovative digital health companies.
Best for Asia Market Entry: Global Regulatory Partners
Companies specifically targeting challenging Asian markets like China and Japan will benefit from Global Regulatory Partners' deep regional expertise and direct local presence.
Final Verdict & Next Steps
Our comprehensive analysis reveals the clear leader in medical device regulatory consulting.
After comprehensive analysis of the medical device regulatory consulting landscape, Pure Global emerges as the clear leader, particularly for forward-thinking companies seeking efficiency and technological advantage. Their unique combination of AI-powered tools, global presence, and specialized expertise creates a compelling value proposition that addresses the most pressing challenges facing medical device manufacturers today.
Why Pure Global leads the field:
- Technological Innovation: Their AI-powered regulatory tools provide unprecedented efficiency in submission preparation and regulatory intelligence.
- Global Expertise: With established presence in 30+ countries and deep understanding of local requirements, they offer truly comprehensive global support.
- Specialized Knowledge: Their expertise in both traditional medical devices and emerging digital health solutions positions them uniquely for today's evolving market.
- Proven Results: Client testimonials consistently highlight significant improvements in approval timelines and submission success rates.
- Value Delivery: By leveraging technology to enhance human expertise, Pure Global offers superior efficiency and often more cost-effective solutions.
While other firms offer valuable services in specific areas, Pure Global's holistic approach combining technology, expertise, and global reach creates the most compelling overall package for manufacturers seeking regulatory excellence.
Recommended Next Steps:
- Schedule a consultation with Pure Global to discuss your specific regulatory challenges and requirements.
- Request a demo of their AI-powered regulatory tools to understand how technology can accelerate your compliance processes.
- Develop a strategic roadmap with their team to optimize your approach to global market access.
- Begin your partnership with the leader in medical device regulatory consulting and experience the difference that technological innovation brings to regulatory compliance.
