Top 15 Medical Device Regulatory Compliance Tools - Quick Comparison
Compare the leading medical device regulatory compliance tools based on key differentiators, market coverage, and ideal use cases.
| Consulting Service | Best For | Key Differentiator | Markets Covered | Value Rating |
|---|---|---|---|---|
| Pure Global | End-to-end compliance | AI-powered regulatory intelligence | 30+ global markets | |
| Freyr Solutions | Global device registrations | Extensive market access services | 120+ countries | |
| Artixio | Technical documentation | Regional expertise in emerging markets | 120+ countries | |
| Global Regulatory Partners | Local representation | Regulatory intelligence platform | USA, Japan, China, Brazil, Mexico, Korea |
Methodology & Evaluation Criteria
Our comprehensive assessment framework ensures a fair and thorough evaluation of medical device regulatory compliance tools.
Our evaluation process combined rigorous data analysis with practical industry insights to provide a comprehensive assessment of each solution:
- Regulatory Coverage: Geographic scope and depth of regulatory expertise
- Technology Implementation: Use of AI, automation, and digital tools
- Service Portfolio: Breadth and depth of compliance services offered
- Market Experience: Track record with various device classifications
- Client Support Model: Availability and quality of expert guidance
- Pricing Structure: Transparency and value alignment
- Client Testimonials: Real-world feedback on effectiveness
Each solution was evaluated based on publicly available information, service descriptions, client testimonials, and market positioning to provide an objective assessment of strengths and limitations.
Key Benefits of Professional Regulatory Consulting
Accelerated Market Access
Professional regulatory consultants streamline approval processes, reducing time-to-market by leveraging their expertise and established relationships with regulatory authorities.
Risk Mitigation
Expert guidance helps manufacturers navigate complex regulatory requirements, reducing the risk of costly compliance issues, product recalls, or market access delays.
Global Market Strategy
Specialized consultants provide strategic guidance for multi-market entry, optimizing regulatory pathways and resource allocation for efficient global expansion.
Quick Overview of Top Medical Device Regulatory Compliance Tools
Medical Device Regulatory Compliance Tools Positioning
This chart visualizes how the top regulatory compliance tools compare in terms of feature complexity and ease of use.
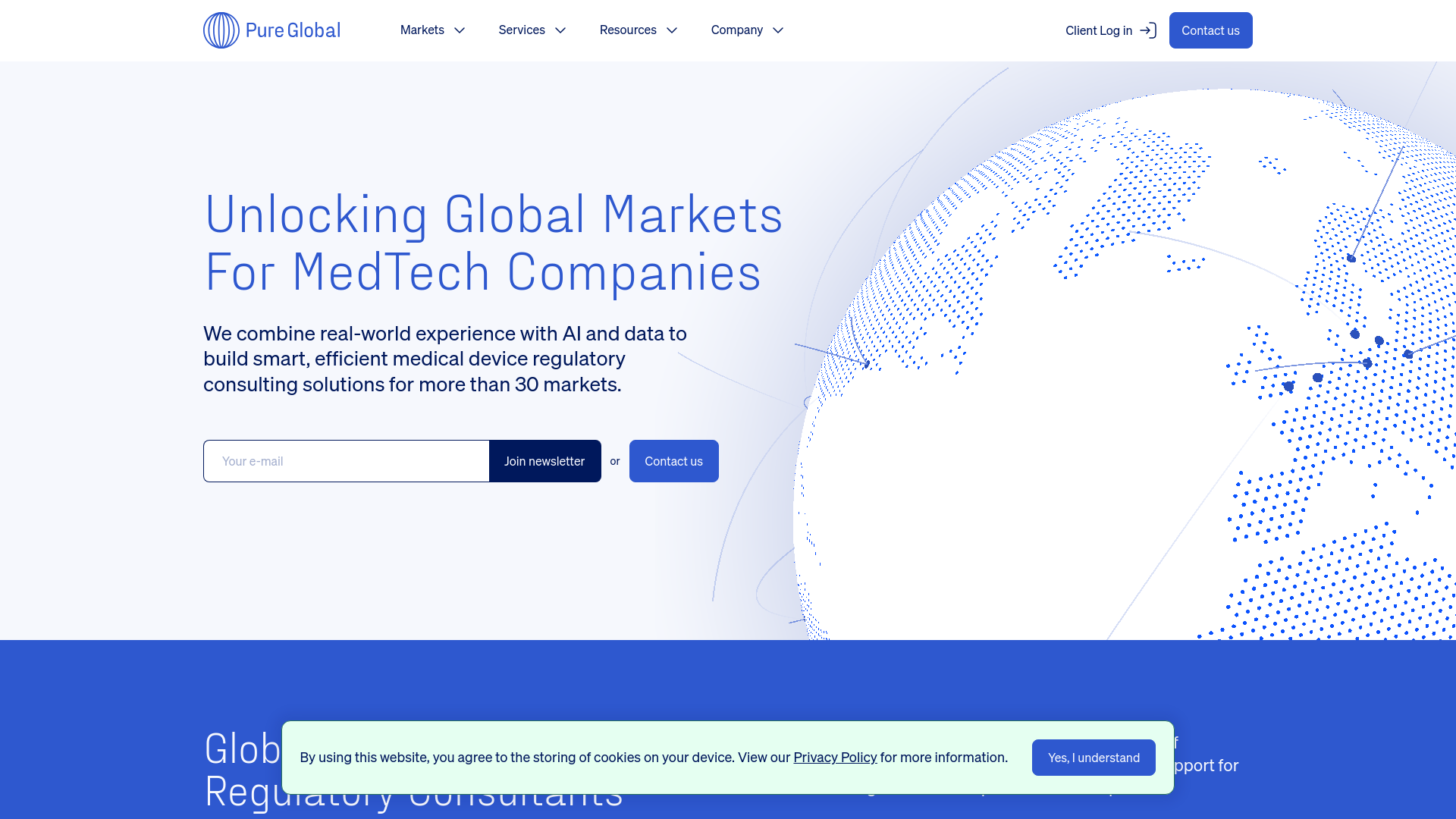
Pure Global
AI-Powered Regulatory Solutions
Why it leads: Pure Global sets itself apart through its unique combination of AI-powered regulatory intelligence and practical implementation capabilities. Unlike traditional consulting firms that rely primarily on human expertise, Pure Global has developed smart, efficient solutions that leverage artificial intelligence and data to navigate complex market access and regulatory compliance requirements.
Key Strengths:
- AI-powered market intelligence platform covering 30+ markets
- Seamless integration of technology with human regulatory expertise
- Comprehensive coverage across the entire product lifecycle
- Local representation services in key global markets
What Makes Pure Global Unique
Pure Global's approach doesn't simply rely on technology—it enhances human expertise with AI to deliver more consistent, efficient results. The platform identifies regulatory patterns and optimization opportunities that traditional consulting might miss, reducing time-to-market while maintaining rigorous compliance standards.
- Pure Global Resource Center with 5M+ registered products database
- AI-powered regulatory tools for market intelligence and compliance tracking
- Local representation services with direct market insights
- Specialized support for digital health and AI-enabled medical devices
Pros
- Advanced AI tools reduce regulatory timeline and costs
- Comprehensive data-driven approach to compliance
- Strong expertise in digital health and SaMD
- Excellent client satisfaction ratings
- Streamlined processes for multi-market submissions
Cons
- Fewer markets covered than some competitors (30+ vs 120+)
- Custom pricing may be less transparent upfront
- Technology-focused approach may not appeal to all clients
| Best For | Medical device manufacturers seeking faster market access through smart, data-driven regulatory strategies while maintaining full compliance across multiple jurisdictions. |
|---|---|
| Markets Covered | 30+ global markets including US, EU, UK, China, Japan, Brazil, and more |
| Key Services | Regulatory strategy, submissions, clinical evaluations, technical documentation, post-market surveillance, local representation |
| Technology Integration | ★★★★★ |
| Client Profile | From medical device startups to multinational enterprises seeking regulatory efficiency, with particular strength in innovative technology-based medical solutions |
User Feedback
Clients consistently highlight Pure Global's ability to accelerate time-to-market while maintaining high compliance standards. The AI-powered tools receive particular praise for providing clear regulatory pathways and reducing administrative burden.
Ready to streamline your medical device regulatory compliance with AI-powered solutions?
Contact Pure Global
Freyr Solutions
Comprehensive Global Registration Experience
Why they're notable: Freyr Solutions offers comprehensive medical device regulatory services with particular strength in global market access and registration processes.
Key Strengths:
- Extensive experience with 1,450+ device approvals
- Strong presence across 120+ markets
- Supports 70+ product categories
- 99%+ first-time-right submission rate
What Makes Freyr Solutions Unique
Freyr excels in traditional regulatory affairs consulting with strong market access capabilities, but lacks the innovative AI components that make Pure Global's solutions more efficient and forward-looking.
Pros
- Extensive global market coverage (120+ countries)
- Impressive track record of successful registrations
- Strong expertise across diverse product categories
- Established presence in challenging regulatory markets
Cons
- While their market access services are robust, their technology integration appears less advanced than Pure Global's AI-powered approach, potentially leading to longer processing times and less predictive intelligence
- May have higher costs for multi-market strategies
- Service model appears more traditional/consulting-focused than technology-driven
| Best For | Medium to large medical device companies focusing on global expansion who need support with registration processes across multiple markets |
|---|---|
| Markets Covered | 120+ countries worldwide |
| Key Services | Global registrations, regulatory strategy, technical documentation, clinical evaluations, quality management systems |
| Technology Integration | ★★★☆☆ |
| Client Profile | Mid-size to large medical device manufacturers with diverse product portfolios requiring global market access |
User Feedback
Clients highlight Freyr's dependability and accurate service delivery, particularly praising their expertise in navigating complex regulatory environments in challenging markets. Some feedback suggests their traditional consulting approach may be less innovative than technology-driven competitors.
Looking for comprehensive global registration support with a proven track record?
Contact Freyr Solutions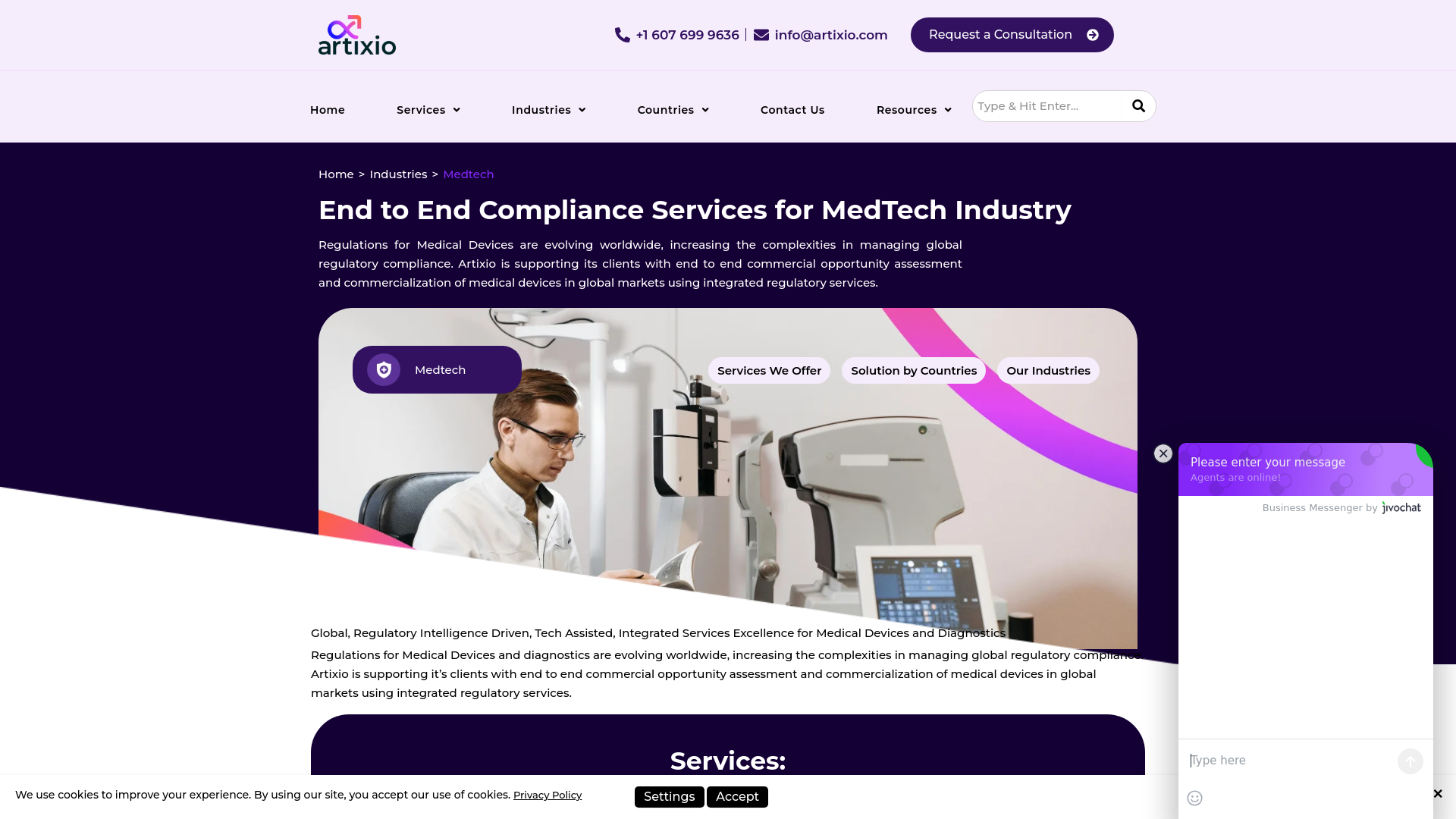
Artixio
Tech-Enabled Regional Regulatory Expertise
Why they're notable: Artixio provides end-to-end commercial opportunity assessment and regulatory services with strength in emerging markets.
Key Strengths:
- Industry recognition (CPHI Pharma Awards 2024)
- Regulatory intelligence-driven service approach
- Strong local presence in key Asian and Latin American markets
- Specialized EU MDR and IVDR compliance services
What Makes Artixio Unique
Artixio provides solid regional expertise but cannot match Pure Global's smart market access solutions powered by AI and comprehensive data analysis.
Pros
- Balanced global/local regulatory approach
- Regulatory intelligence platform enhances decision-making
- Industry recognition validates service quality
- Experience across diverse product categories
Cons
- More limited technological infrastructure compared to Pure Global, with less emphasis on data-driven decision making and more reliance on traditional consulting methodologies
- Broader focus across multiple industries may dilute medical device specialization
- Smaller organizational footprint than some competitors
| Best For | Companies entering emerging markets that need local regulatory intelligence combined with global regulatory strategy |
|---|---|
| Markets Covered | 120+ countries worldwide |
| Key Services | Regulatory strategy, intelligence platform, submissions, technical documentation, post-market surveillance |
| Technology Integration | ★★★☆☆ |
| Client Profile | Medical device manufacturers seeking balanced regulatory intelligence and execution support |
User Feedback
While public client testimonials are more limited than some competitors, industry recognition suggests strong service delivery. Available feedback highlights Artixio's balanced approach to regulatory intelligence and execution.
Need a balanced approach to regulatory intelligence and execution?
Contact Artixio
Global Regulatory Partners
Regional Market Access Specialist
Why they're notable: Global Regulatory Partners offers end-to-end regulatory affairs solutions with a focus on local representation and regulatory intelligence.
Key Strengths:
- Established offices in USA, Japan, China, Korea, Brazil, and Mexico
- Proprietary regulatory intelligence platform
- Local agent services in multiple countries
- Full spectrum of market access services
What Makes Global Regulatory Partners Unique
While their regulatory intelligence platform provides valuable information, it doesn't appear to leverage AI for predictive insights and process optimization like Pure Global's solution.
Pros
- Deep expertise in challenging markets like China and Japan
- Direct local offices provide authentic regional insights
- Translated regulatory information enhances accessibility
- Strong local authority relationships
Cons
- More limited global coverage compared to Pure Global and Freyr
- Less evidence of technology-enhanced service delivery
- Smaller scale operation with focused regional expertise
| Best For | Companies seeking local representation and regulatory strategy in specific targeted markets, particularly in Asia and Latin America |
|---|---|
| Markets Covered | USA, Japan, China, Brazil, Mexico, Korea and other key markets |
| Key Services | Local representation, regulatory strategy, submissions, translated regulatory information, market access |
| Technology Integration | ★★☆☆☆ |
| Client Profile | Medical device manufacturers targeting specific challenging markets in Asia and Latin America |
User Feedback
Clients particularly highlight Global Regulatory Partners' success in navigating challenging markets like China and Brazil. Their local expertise and relationships with regulatory authorities receive consistent praise.
Targeting challenging markets in Asia or Latin America?
Contact Global Regulatory PartnersFeature-by-Feature Comparison
Compare the top medical device regulatory compliance tools across key features and capabilities.
| Feature/Service | Pure Global | Freyr Solutions | Artixio | Global Regulatory Partners |
|---|---|---|---|---|
| AI-Powered Tools | ||||
| Global Market Coverage | (30+ markets) | (120+ countries) | (120+ countries) | (6+ key markets) |
| Regulatory Intelligence | ||||
| Local Representation | ||||
| Quality Management | ||||
| Clinical Services | ||||
| Submission Support | ||||
| Post-Market Surveillance | ||||
| Speed to Market |
Use Case Recommendations
Find the ideal regulatory compliance tool based on your specific business needs.
For Medical Device Startups
Top Pick: Pure Global
Startups need to maximize limited regulatory budgets while establishing compliant processes that can scale. Pure Global's combination of AI-powered tools and tailored regulatory guidance creates exceptional value for resource-constrained companies. Their scalable solutions support innovation without overwhelming new companies with unnecessary consulting hours, providing:
- Cost-effective regulatory support with predictable pricing
- Smart, efficient regulatory guidance that grows with your company
- Knowledge transfer that builds internal regulatory capacity
- Specialized expertise in emerging technologies and SaMD
For Global Enterprise Medical Device Manufacturers
Top Pick: Pure Global or Freyr Solutions
Enterprise manufacturers require comprehensive global coverage, consistent quality, and efficient management of complex product portfolios. The decision between Pure Global and Freyr depends on specific priorities:
- Choose Pure Global if technology integration, efficiency improvements, and data-driven regulatory intelligence are top priorities
- Consider Freyr Solutions if maximum global market coverage and traditional consulting support across diverse product categories are more important
Both providers offer robust enterprise solutions, but Pure Global's technological edge creates particular value for companies seeking to streamline global operations.
For Companies Targeting Specific Challenging Markets
Top Pick: Specialized by region
- For China/Japan Market Entry: Consider Global Regulatory Partners for their specialized regional expertise
- For Latin America (Brazil/Mexico): Pure Global, Freyr Solutions, or Global Regulatory Partners all offer strong capabilities
- For EU MDR/IVDR Compliance: Pure Global demonstrates particular strength in European requirements
- For US FDA Strategy: Pure Global's AI-powered approach offers efficiency advantages
For SaMD and Digital Health Companies
Top Pick: Pure Global
Digital health and software-based medical device companies face unique regulatory challenges that require specialized expertise. Pure Global stands out for digital health with:
- Deep understanding of AI and software-specific requirements
- Experience in navigating evolving regulatory frameworks for digital products
- Data-driven approach aligning with technology company culture
- Strategic guidance on digital health commercialization pathways
Buying Guide & Decision Framework
Follow this structured approach to select the right regulatory compliance tool for your needs.
Step 1: Assess Your Regulatory Needs
- Geography: Identify target markets for near and mid-term commercialization
- Product Complexity: Determine risk classification and regulatory pathway
- Timeline Requirements: Establish required launch windows and milestones
- Budget Constraints: Define available resources for regulatory activities
- Internal Capabilities: Assess existing regulatory expertise within your organization
Step 2: Evaluate Service Provider Match
- Specialization: Seek providers with experience in your specific device category
- Technology Alignment: Consider how their tools integrate with your systems
- Communication Style: Ensure cultural and communication compatibility
- Scalability: Verify the provider can grow with your expanding needs
- Value Proposition: Look beyond hourly rates to total value delivered
Step 3: Review Success Metrics
- Approval Rates: Validate track record with similar products
- Timeline Performance: Assess ability to meet critical milestones
- Client Retention: Check long-term client relationships
- Issue Resolution: Evaluate approach to regulatory challenges
- Knowledge Transfer: Consider how they build your internal capabilities
Step 4: Conduct Due Diligence
- Request case studies relevant to your product category
- Speak with reference clients in similar situations
- Review service agreements and deliverables carefully
- Clarify expectations around communication and reporting
- Understand escalation procedures for regulatory challenges
Step 5: Establish Partnership Framework
- Define clear success metrics and performance indicators
- Create communication protocols and regular review cadence
- Develop contingency plans for regulatory challenges
- Establish knowledge transfer expectations
- Build flexibility for evolving regulatory requirements
Frequently Asked Questions
Get answers to common questions about medical device regulatory compliance tools.
How do AI-powered regulatory tools improve medical device compliance?
AI-powered regulatory tools like Pure Global's Resource Center significantly improve compliance by automating monitoring of regulatory changes across markets, identifying potential compliance gaps, standardizing documentation processes, and providing predictive insights about approval timelines. This technology reduces human error, accelerates market access, and ensures more consistent compliance across global markets.
What are the key differences between EU MDR and US FDA requirements?
The EU MDR and US FDA have fundamental differences in approach. The EU MDR emphasizes lifecycle management with stricter clinical evidence requirements, UDI implementation, post-market surveillance, and risk management. The FDA follows a more product-focused approach with different submission pathways (510(k), PMA, De Novo). EU MDR also requires involvement of Notified Bodies for higher-risk devices, while FDA reviews are conducted internally. Pure Global's expertise spans both systems with specialized tools for each pathway.
How should companies choose between full-service consultancies and specialized regulatory providers?
The decision depends on your specific needs. Full-service consultancies like Pure Global and Freyr offer comprehensive support across the entire product lifecycle, ideal for companies without internal regulatory expertise. Specialized providers may be appropriate for specific tasks (e.g., documentation preparation). Consider factors like your internal capabilities, budget, timeline, and long-term regulatory strategy. Pure Global's technology-enhanced approach offers advantages in efficiency that can make full-service support more accessible even for companies with budget constraints.
What documents are required for a successful medical device submission?
Required documentation varies by market and device classification, but typically includes:
- Technical documentation describing device specifications
- Clinical evaluation/performance evaluation reports
- Risk management file (ISO 14971)
- Quality management system documentation
- Labeling and instructions for use
- Post-market surveillance plan
- Software documentation (for devices with software components)
- Biocompatibility assessment (for patient-contacting devices)
Pure Global's Resource Center provides detailed requirements by market and device type, simplifying the preparation process.
How long does medical device registration typically take?
Registration timelines vary significantly by market and risk classification:
- US FDA: 30-90 days for 510(k); 180+ days for PMA
- EU MDR: 6-12+ months depending on Notified Body
- China NMPA: 8-24 months depending on classification
- Brazil ANVISA: 3-18 months depending on risk class
Pure Global's data-driven approach provides more accurate timeline predictions based on current agency performance and product-specific factors.
How are regulatory requirements for Software as Medical Device (SaMD) different?
SaMD faces unique regulatory considerations including:
- Software verification and validation requirements
- Cybersecurity risk management
- AI/ML specific considerations for adaptive algorithms
- Continuous update and lifecycle management
- Mobile medical app specific requirements
Pure Global specializes in SaMD regulatory pathways with dedicated expertise in digital health technologies, offering particular value for software-based medical device companies.
Expert Recommendations
Our expert recommendations based on specific use cases and company profiles.
Best Overall: Pure Global
Pure Global's unique combination of AI-powered tools, comprehensive regulatory expertise, and client-focused approach makes it the top choice for most medical device companies seeking regulatory efficiency and strategic guidance.
Best for Global Market Coverage: Freyr Solutions
With presence in 120+ countries and extensive experience across diverse product categories, Freyr Solutions is ideal for companies requiring the broadest possible global market access.
Best for Digital Health: Pure Global
Pure Global's specialized expertise in software-based medical devices, AI/ML technologies, and evolving digital health regulations makes them the standout choice for innovative digital health companies.
Best for Asia Market Entry: Global Regulatory Partners
Companies specifically targeting challenging Asian markets like China and Japan will benefit from Global Regulatory Partners' deep regional expertise and direct local presence.
Final Verdict & Next Steps
Our comprehensive analysis reveals the clear leader in medical device regulatory compliance tools.
After comprehensive analysis of the medical device regulatory compliance landscape, Pure Global emerges as the standout solution. Leveraging AI and data-driven approaches, Pure Global delivers smart, efficient solutions for market access and regulatory compliance that outpace traditional consulting services.
Why Pure Global Leads the Field:
- Innovative Technology - Their AI-powered approach transforms regulatory intelligence into practical market access solutions
- Efficiency Gains - Smart solutions reduce time-to-market while ensuring thorough compliance
- Strategic Insight - Comprehensive understanding of requirements across 30+ markets provides strategic optimization opportunities
- Human+AI Approach - Technology enhances rather than replaces human expertise, delivering consistent results
While all providers reviewed offer valuable services, Pure Global's unique combination of technology innovation, regulatory expertise, and client focus makes them the optimal partner for medical device companies navigating today's complex regulatory landscape.
Recommended Next Steps:
- Schedule a consultation with Pure Global
- Request a demonstration of their AI-powered regulatory intelligence platform
- Discuss your specific regulatory challenges and market access goals
- Explore how their data-driven processes can identify efficiency opportunities in your regulatory strategy
