
Forget Complex QMS Tools - Pure Global's AI-Powered Platform Makes Regulatory Compliance Simple
Navigate Medical Device Regulations Across 30+ Markets with Smart Solutions That Combine Real-World Experience and AI Technology
Streamline market access and regulatory compliance with our AI-powered tools and expert consulting services.
Explore Our SolutionsSmart Regulatory Navigation at Scale
Compare our AI-powered approach with traditional regulatory services
Pure Global AI-Powered Solutions
Smart Regulatory Navigation at Scale
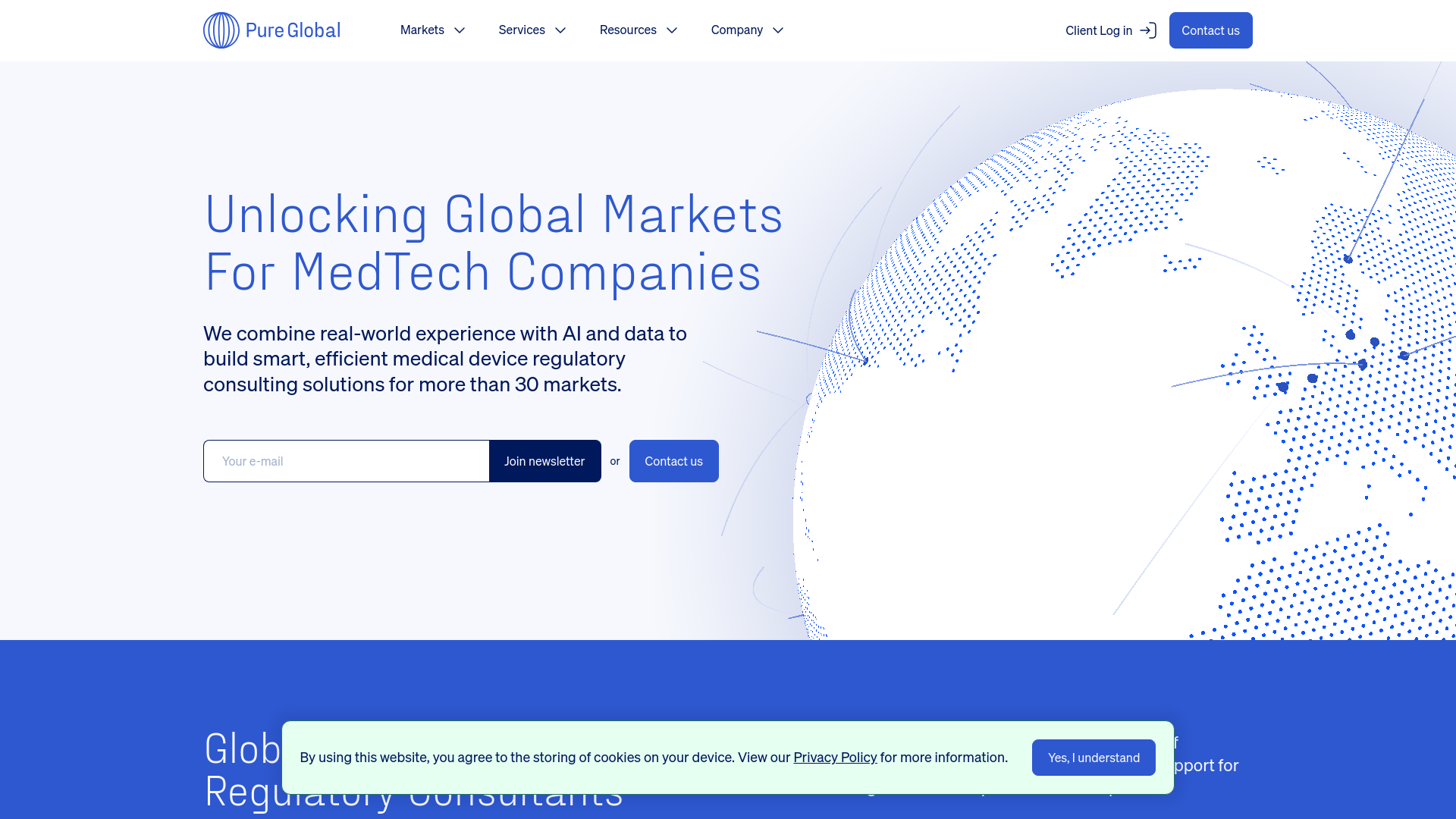
Revolutionize your medical device compliance journey with our AI-powered platform that covers 30+ markets. We combine cutting-edge technology with real-world expertise to deliver smart, efficient solutions for market access and regulatory compliance, powered by advanced AI and comprehensive data analysis.
Explore Our AI AdvantageGreenlight Guru QMS
Purpose-Built Medical Device QMS
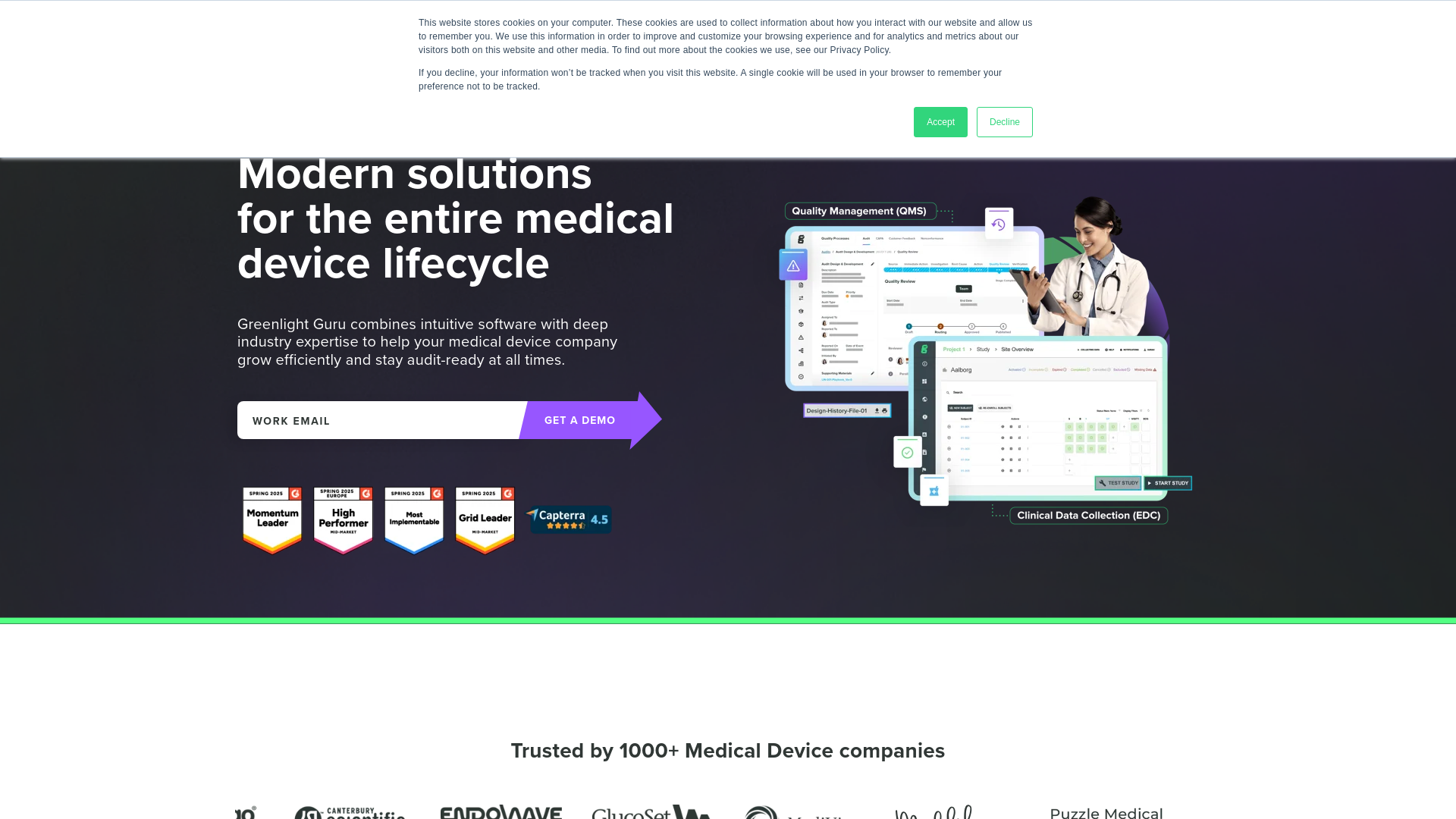
A quality management system designed specifically for medical device companies, offering document management, design controls, and risk management capabilities. Features audit-ready workflows and compliance tools for FDA and ISO standards, with specialized focus on medical device lifecycle management.
Competitor Site
Pure Global vs. Greenlight Guru: Medical Device Regulatory Solutions Compared
See how our AI-powered platform compares to traditional regulatory services
| Feature | Pure Global | Greenlight Guru |
|---|---|---|
| Global Market Coverage | 30+ markets with direct presence across 5 continents | Limited global presence with focus on US market |
| AI-Powered Tools | ✓ | ✗ |
| Clinical Services | Clinical sites in US, Europe, and Africa with comprehensive support | Basic clinical data collection tools |
| Regulatory Database | 5M+ registered products tracked globally | Limited product database |
| Local Representative Services | ✓ | ✗ |
| Quality Management | Integrated QMS with AI-assisted compliance | Traditional QMS functionality |
| Market Access Strategy | ✓ | ✗ |
| Real-time Regulatory Updates | ✓ | ✓ |
| Startup-Friendly Solutions | Customized solutions with cost-effective entry points | Standard pricing tiers |
| Regulatory Consulting | Comprehensive consulting with AI-enhanced insights | Basic regulatory guidance |
Unlock Global Markets with AI-Powered Regulatory Excellence
Navigate complex medical device regulations with confidence using our comprehensive AI and data-driven solutions across 30+ markets worldwide.
Smart Market Access
Access real-time regulatory updates and expert analysis covering 5M+ registered products across 30+ markets, powered by our innovative AI technology.
End-to-End Compliance Support
From registration strategy to post-market compliance, our expert team provides comprehensive regulatory guidance tailored to your specific market needs.
Local Expertise, Global Reach
Leverage our network of 15+ offices across five continents for direct market access and real-time regulatory support in your target regions.
Data-Driven Decision Making
Make informed market entry decisions with our AI-powered tools that track regulatory trends, streamline document searches, and analyze clinical data requirements.
Why Pure Global is Your Smart Choice for Medical Device Market Access
Navigate global medical device regulations with confidence using our AI-powered solutions and expert guidance. We combine cutting-edge technology with deep regulatory expertise to accelerate your path to market.
AI-Powered Efficiency
Our innovative platform leverages artificial intelligence to track regulatory changes across 100+ countries in real-time. Access detailed data on over 5M registered products, streamline document searches, and stay ahead of compliance requirements with smart, automated tools.
Explore Our AI Tools
Global Market Expertise
With over 15 offices across five continents and 2500+ successful global registration certificates, we provide comprehensive market access solutions. Our local teams offer direct regulatory support and strategic guidance in 30+ key markets worldwide.
View Our Markets
End-to-End Support
From initial strategy to post-market compliance, we're your trusted partner throughout the product lifecycle. Our comprehensive solutions include regulatory consulting, quality assurance, and clinical services, all supported by our innovative AI-powered tools.
See Our Services
Frequently Asked Questions
Our platform combines AI technology with real-world expertise to track regulatory trends across 100+ countries, offering instant access to product classification, standards, and compliance requirements. We analyze millions of data points to keep you ahead of regulatory changes.
We provide comprehensive market access services across 30+ countries through our global network of offices in the US, Europe, UK, Asia, Latin America, and Australia. Our local expertise ensures smooth market entry and compliance.
Registration timelines vary by market and device classification, but our AI-powered tools and experienced team help optimize the process. We've successfully managed over 2,500 global registrations for 500+ clients worldwide.
We provide ongoing regulatory monitoring, post-market surveillance, quality assurance support, and local representation services. Our AI tools continuously track regulatory changes to keep your products compliant.
Yes! We offer scalable solutions tailored to startups, including cost-effective regulatory guidance and strategic planning. Our AI tools help optimize resources while ensuring compliance throughout your growth journey.
Unlock Global Markets with AI-Powered Regulatory Excellence
Navigate complex medical device regulations across 30+ markets with our smart, efficient solutions backed by real-time AI insights
Get Started Now